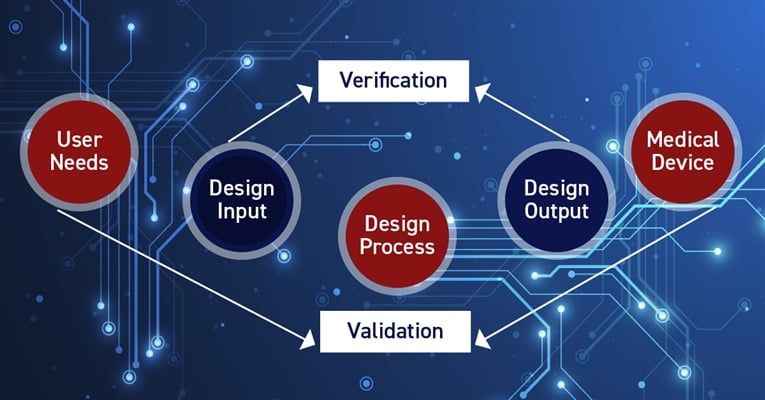
The FDA has published a guide to help medical device companies quickly become “recall ready,” including how voluntary product recalls can be done faster and more efficiently by having a digital thread (PLM) in place.
The US Food and Drug Administration (FDA) recently published an industry guidance for recalls to help companies, including medical device manufacturers, quickly and effectively correct or remove products in violation of FDA laws from the market. The guidance outlines the steps companies should take to develop recall policies and procedures before a recall is necessary. With a focus on training, planning, and record-keeping, recall-ready companies can quickly and effectively remove products from the market and limit the public’s exposure to risk. The FDA has the authority to require recalls of certain products, including medical devices.
A voluntary recall is an action taken by a company to correct a product in violation or remove it from the market. The new guidance explains how proper product coding and distribution records help facilitate faster, more accurate recall actions. It encourages companies to use electronic communications to quickly identify and provide certain product information when alerting consignees and the public about a voluntary recall.
Recall levels at a record high
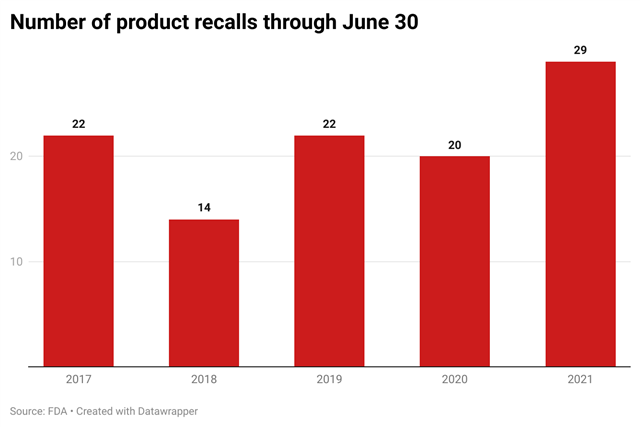
The publication of the guidance reflects the agency’s response to the growing number of product recalls over the last five years. The number of recalls issued during the first half of 2021, however, was higher than the number of recalls issued during the first half of each of the previous five years.
Among the most serious of these recalls is related to HVAD pump implant kits. The first 3 months of 2022 have so far resulted in 12 medical device product recalls, including the recalls of CPAP and BiPAP ventilator machines.
From this set of recalls, device design is the most common FDA-determined cause. Faulty design may stem from insufficient design control during the product development phase. Examples of device design issues include components that do not stay in place, interfaces suspect to user misinterpretation, and inaccurate assays or measuring components.
Managing a nationwide recall is already challenging and adding the logistics of coordinating international measures can become extremely difficult if well designed systems are not in place.
The FDA estimate is that if the industry could address these problems jointly with the agency – including manufacturers improving their design control procedures - it is possible to prevent as many as 90% of recalls each year.
Becoming recall ready
It is critical for manufacturers and their product distribution chains to be “recall ready,” according to the FDA. It recommends that a manufacturer make the following general preparations in advance of a potential recall:
- Identify relevant products and use adequate product coding
- Establish a recall communications plan
- Identify appropriate personnel & train them on their responsibilities
- How a digital thread can help prepare for product recalls
- Identify relevant products and use adequate product coding
Product Identification & Unique Device Identification capabilities like those found in Aras Innovator help companies create individual serialised products and parts or batch/lot identification of products and the identification of receiving customer. With the digital thread, manufacturers can easily obtain customer details for communication purposes should a product recall be needed.
Many medical devices must bear a Unique Device Identifier (UDI) on their labels and device packages. The UDI and serial numbers can easily be tracked and located with the digital thread to find all required documentation or information. The coding used should allow for the identification of the production and control data created for each lot, batch, or unit.
Establish a recall communications plan
A critical element to any product recall strategy is a well-developed communications plan that answers the questions who, what, when, where, and how. The QMS Corrective Action Plan inside Aras Innovator can be used during a recall to identify, capture, and execute the associated activities needed to enact a recall, including identifying, analysing, and actioning any deviations, rework orders, change notices to both correct and/or prevent any additional problems.
Written recall procedures can be stored, approved, and released with the digital thread. The recall procedures can be easily found attached to the correct product.
Identify appropriate personnel & train personnel on their responsibilities
The learning management system and the associated training record can be used to ensure that personnel who have been identified to perform recall activities have been trained on a regular basis, so they have a thorough understanding of the recall procedures they are being asked to perform.
During a recall
Traceability of affected product in the production network is vital to minimize the impact of a recall. The key factor is quick access to accurate and relevant information. The digital thread enables fast traceability of all results and metadata from any suspect product back through all stages of production to initial test results. This enables operators to see which batches could be affected and take appropriate action to contain the issue.
For this reason, it is essential to have a system in place which will enable you to immediately identify which batch, and which finished products, might be implicated.
Because recalls can affect the entire supply chain, including downstream suppliers, wholesalers, or vendors, the FDA recommends that companies develop recall procedures to quickly inform their entire distribution chain, so consignees can rapidly identify affected lots and recall downstream products when necessary.
This might seem obvious, but recent events still show significant issues communicating recalls to customers and suppliers.
For more information on how to improve your design control procedures, be sure to check out our webinar, Mastering Design Control with Medical Device PLM.
For more details on bolstering your recall readiness, be sure to review Initiation of Voluntary Recalls Under 21 CFR Part 7, Subpart C.